The race to develop a safe and effective vaccine against COVID-19 is on, and it has everyone talking. This is the third of my three-part series on the fundamentals of immunization.
Part 1 of the series reviewed and clarified fundamental terms that are central to the conversation and provided answers to some of the most commonly asked questions.
Part 2 established a solid foundation of the currrent science and provided insight into the regulatory realities that control a vaccine’s path to market (including the routine development and approval processes), the impact vaccines have had on our society, and provided a glimpse into the future of vaccine technology.
We will begin this third and final segment by highlighting the non-routine elements put into place to speed the development and apporval path for COVID therapeutic treatments and prevenative vaccines. This final installment will also look closely at the leading candidates in the race for safe and effective COVID-19 vaccines and therapeutics, as well as the technologies being utilized to shephard us to the end of this pandemic.
Note: There have been two vaccine candidates already approved for distribution in Russia by the Ministry of Health of the Russian Federation, but this blog will focus on the candidates hoping to gain USFDA approval.
Public and Private Partnerships, Working at Warp Speed
The aggressive worldwide research and development effort began in January of 2020 when the genetic sequence of SARS-CoV-2, the coronavirus that causes COVID-19, was identified and published. The scale of the humanitarian and economic impact of the COVID-19 pandemic compelled researchers, manufacturers and the world’s governments to work together to minimize delays and accelerate the pace of development and delivery of safe and effective therapeutics and vaccines, in huge quantities.
The success of these collaborations is as unprecedented as the scale of the pandemic, with the first American COVID-19 vaccine candidate entering clinical trials just two months after the genetic information had been released.
The most significant partnership we have seen surface as a result of this pandemic, has been the initiation of the U.S. public-private partnership named Operation Warp Speed (OWS). OWS was launched in order to accelerate the steps needed to develop, test, approve, manufacture, and distribute COVID-19 vaccines, therapeutics, and diagnostics.
The initiative involved the investment of public money and resources, the transparent sharing of standardized methods and information between researchers and companies, a streamlined approval process which allowed steps to happen simultaneously, and the use of government agencies to aid in the distribution of the approved products. All of these actions were taken in order to deliver COVID-19 countermeasures as rapidly as possible, without comprising the products’ efficacy or safety. Prior to this effort, the fastest path ever taken to market vaccine was four years, with most taking 10 – 15 years, with a few extending to 25 years.
The Department of Health and Human Services (HHS) (including the Food and Drug Administration, the Centers for Disease Control and Prevention, the National Institutes of Health, and the Biomedical Advanced Research and Development Authority), and the Department of Defense (DOD) are coordinating the program by working closely with private firms and consultants, as well as additional federal agencies, including, but not limited to, the Department of Agriculture, the Department of Energy, and the Department of Veterans Affairs.
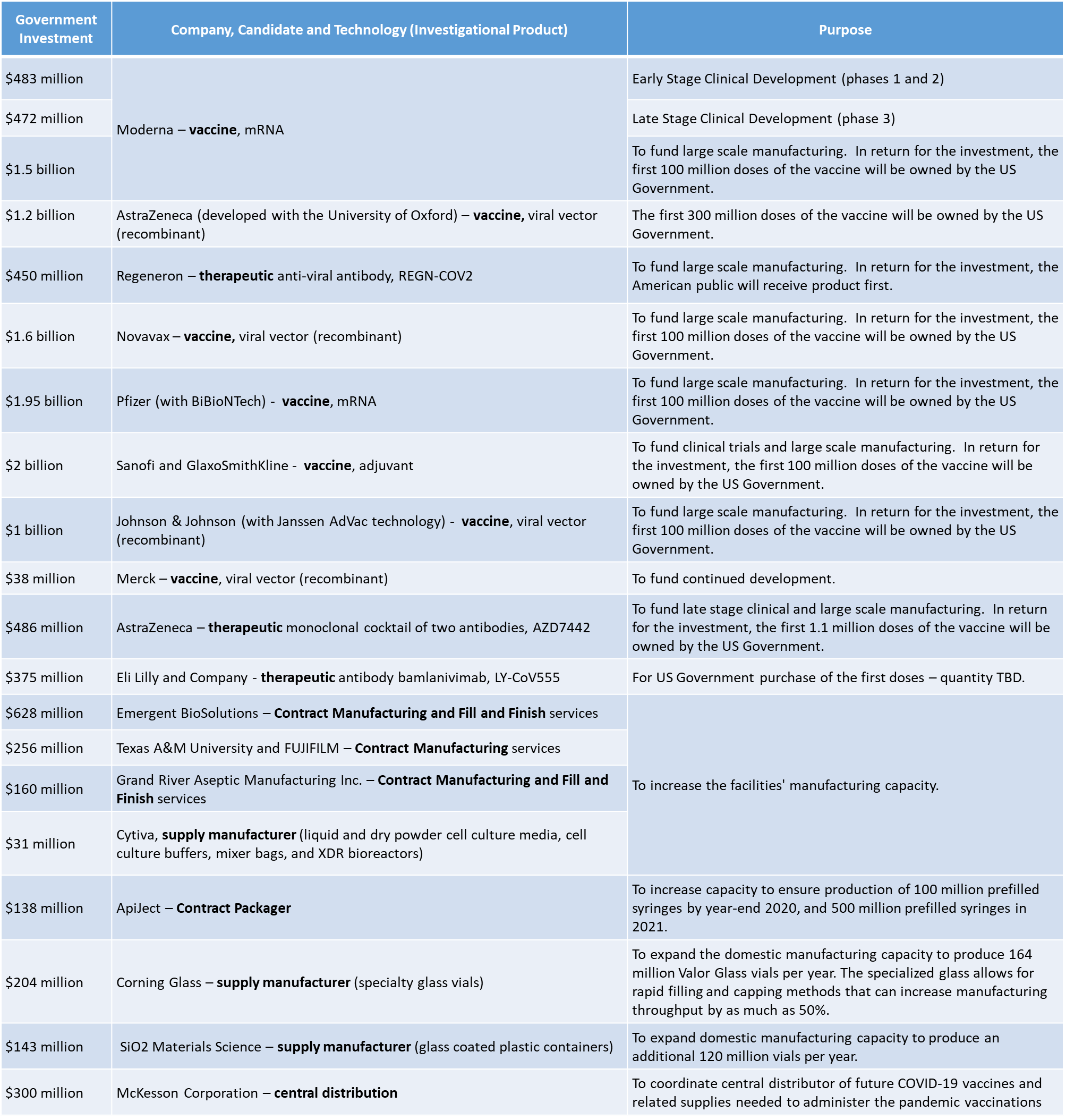
When OWS was launched, more than 125 therapeutic and vaccine candidates arose immediately. Of those, the most promising received substantial financial investments of tax payer dollars, from the U.S. Government. Most of those investments also entitle the U.S. Government to a substantial number of vaccine doses and therapeutic products. The therapeutics and vaccines purchased by the U.S. Government through OWS, are being provided to U.S. citizens at no cost (however, physicians and organizations administering them, may charge the consumer for the cost of administration).
The table below highlights some of the OWS investments led by HHS, in development, testing and production. The HHS investments have been substantial, and well publicized. However, other Operation Warp Speed investments, led by DOD, have also simultaneously focused on developing and funding the infrastructure that will be required to effectively distribute these products nationwide.
Noteworthy Federal Investments into COVID Countermeasures
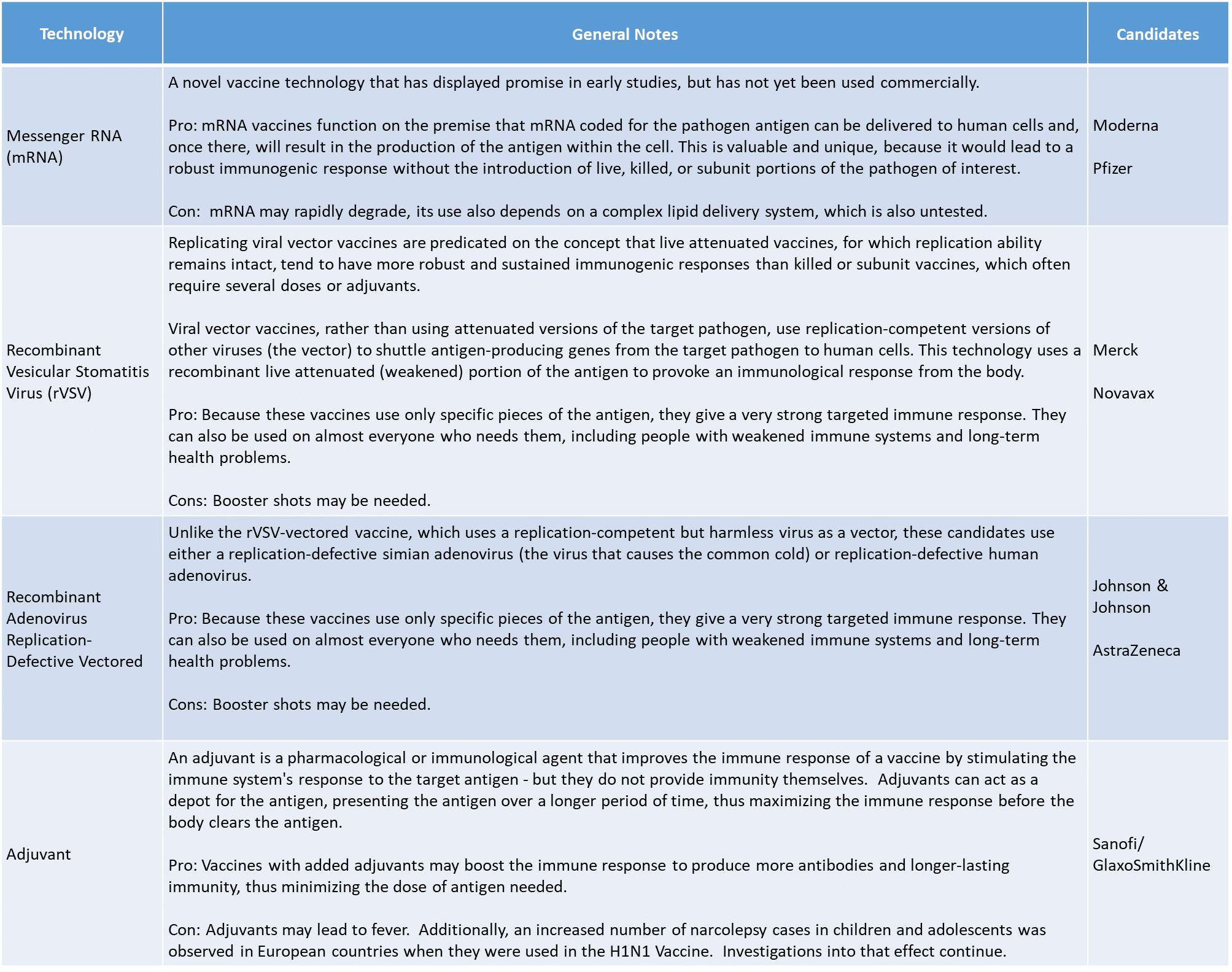
A Closer Look at the Vaccine Technologies in Play
The objective of each of the leading vaccine candidates is to prepare the body’s immune system to recognize and then fight SARS-CoV-2, the virus that causes COVID-19. However, each employs a different strategy to achieve that critical response. The following table groups the technologies into high level categories. Some of these technologies have been used previously, with great success fighting other dangerous pathogens (e.g., Ebola) but others are novel, and have not yet been used on a commercial scale.
While the technologies and advantages differ, making them all available at once, will give health care providers a broad range of options to treat a diverse population.
In Conclusion
COVID-19 has presented the world with one of the greatest health challenges it has ever seen. The world’s scientific community, has responded in kind – inarguably ranking this as one of the most ambitious and collaborative scientific endeavors in history.
The accelerated process will not be able to determine if the first generation of COVID vaccines will provide long lasting immunity, but they will able to demonstrate safety and short-term effectivity.
While the ultimate goal of vaccinology is long lasting immunity, delivered with a single dose, that is not always the case. In fact, the annual flu vaccine varies in effectivity between 20 and 70%, and the CDC reports that during the 2018-19 U.S. flu season, vaccination prevented an estimated 4.4 million influenza infections, 2.3 million medical visits, 58,000 hospitalizations and 3,500 influenza-associated deaths.
The accelerated process continues to enforce FDA’s stringent controls, pausing ongoing trials as needed to allow Independent Review Boards the time they needed to investigate findings. These review pauses make it evident that while accelerated, the process continues to include the scientific scrutiny required to ensure safety and short term efficacy.
To date, several vaccine trials will be completing Phase 3 trials in the coming months, with one of the frontrunners publicly announcing an intent to seek emergency regulatory approval by the end of 2020, with manufacturing capacity, supply chains and central distribution plans in place.
Given this accelerated timeline, the delivery of a safe vaccine that is capable of limiting the impact of the spread and the severity of the disease will be a historic achievement, and hopefully, will herald the end of this pandemic.
The Fundamentals of Immunization, Part 1
The Fundamentals of Immunization, Part 2

Gina Guido-Redden is a quality and regulatory professional with over 25 years of domestic and international industry experience. She is the co-founder and chief operations officer of Coda Corp USA, which provides consultancy services to pharmaceutical, biologics and medical device firms.
Guido-Redden’s history specializes in the areas of facility start up, regulatory compliance and remediation, quality system development, mentorship and training, quality system design, and implementation and management.
She is also a quality systems subject matter expert (SME), frequent seminar presenter, and content contributor to industry publications, including GAMP’s White Paper on Part 11, The Journal of Validation Technology, New Generation Pharmaceuticals, Computer Validation Digest, and MasterControl’s GxP Lifeline. Coda Corp USA is an enterprise partner of MasterControl.

 NAVIGATION
NAVIGATION
