Quality Systems Insights
Test Method Validation, Measurement Systems and Gauge R &R
Guidance documents, international harmonization standards, and industrial standards guide industry members and help us develop a practical ability to apply those understandings to real life situations. Unfortunately, FDA’s The Guidance on Test Method Validation addresses only the validation of chemical and biological laboratory analyses. It does not address the validation of Measurement Systems (methods designed […]
Risk Management: A Primer for Lean Quality Assurance
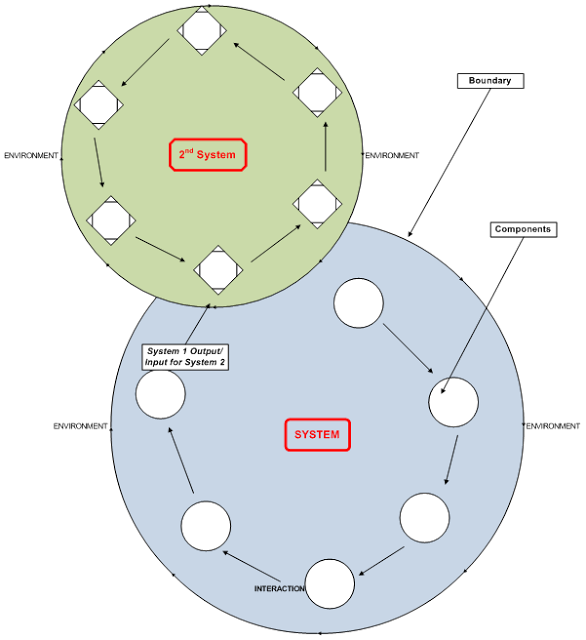
In this blog we will examine what is perhaps the most useful tool in the belt of today’s quality professionals: risk management. Critical Terminology Prior to discussing the topic at hand, I like to provide context for the terminology integral to the concept. The first step on the road to effective system development is alignment […]
Defining Quality with Clarity
If you’re reading this, I’m assuming you earn your living in some sort of Quality Organization, but do you Speak Quality? And if you do, can you speak it fluently, or are you topped out at the conversational level? The reality is, if you’re like many people in Quality Organizations, you may not know. […]
The One Voice Mission: A Quality Opus
I have worked within and around the life sciences community of Quality professionals for more years than I am ready to admit in a public post. Early on in my career, I listened avidly to every experienced person that I worked with, eager to take in everything anyone had to teach me. I was the […]
Understanding, Measuring, and Controlling the total Cost of Quality; The Holy Grail of The Modern Quality System
We decided this month to discuss the concepts, principles, and tools that aim to reduce operating costs while promoting and improving consumer-centric quality. Among this family of principles are those referred to as quality costing analysis models, or “Cost of Quality” (CoQ) models. Industry has been attempting to meet the challenges of measuring and controlling […]
Supplier Quality and the Total Cost of Poor Quality; Understand the Relationship and Avoid the Shortcuts!
During my two decade career, including a decade of consulting, I have integrated with a multitude of organizations, a wide variety of cultures, and a broad spectrum of projects, each with unique objectives, team members, and needs. This diversity of experience has been its own reward and continues to incentivize me to forego the obvious […]
Be Better Than the Best
When I was in college, the robotics club held a fund raiser selling t-shirts that said “INNOVATION” – in all caps and BOLD lettering. I bought one – because I was so sure that someday, I would invent something that would change everything, for everyone. To date, I have not realized that dream. I […]
Measurement System Analysis and Test Method Validation: A Critical Difference
In the previous installment of the blog, we discussed our industries’ need to validate test methods in order to demonstrate that our measurement methods are capable of identifying variances inherent to production methods. Demonstrating suitability must include attempting to quantify or classify the variances that are inherent to the methods. The real trick to proving […]
Should We Improve the Process, or the System We Use to Measure the Process? Measurement Systems Analysis and The Limitations of the Gauge R &R
Should We Improve the Process, or the System We Use to Measure the Process? Measurement Systems Analysis and The Limitations of the Gauge R &R If you’re reading my blog, you probably are required to comply with regulations foreign and/or domestic in order to be successful. What’s more, in order to comply with the regulations, […]
Quality by Design: Part 2
Coda dedicated last month’s installment of the blog to review initiatives undertaken by the Food and Drug Administration that were developed in order to improve regulatory strategies, and agency systems and tools. The improvements we highlighted, among others, were developed and implemented in the hopes of contributing to industries’ efforts to develop new and safe […]
Quality by Design Part 1
In the past, Coda’s blog has covered many initiatives undertaken by the Food and Drug Administration that were developed in order to improve regulatory strategies, and agency systems and tools. These improvements were developed and implemented in the hopes of contributing to industries’ efforts to develop new and safe therapies as quickly as possible and […]
The Difference Between?
The Difference Between? Working in such a tightly regulated and hugely competitive industry and dealing with technologies that can at times be controversial, I expect to hear heated debates or energetic discussions regarding the nature and/or value of regulations, cutting edge technologies, or free market price wars. Because of the nature of our industry, I […]
The Little "c" Isn’t What It Used To Be!
My career began in typical fashion, in the corner of a QC lab working for a mid-sized multi-national pharmaceutical company. But the path from that point forward was anything but typical; in less than a decade, the site was sold 4 times, technology was transferred in and out and then in again, and between owners […]
Think Systems: Cause Quality
November, 2012 was celebrated as “World Quality Month.” ASQ CEO Paul Borawski asked this of industry: “What do you use as the best, most inclusive and illuminating definition of quality?” I followed the trail of replies as they posted, and it quickly became apparent to me just how diverse Mr. Borawski’s audience is, and how […]
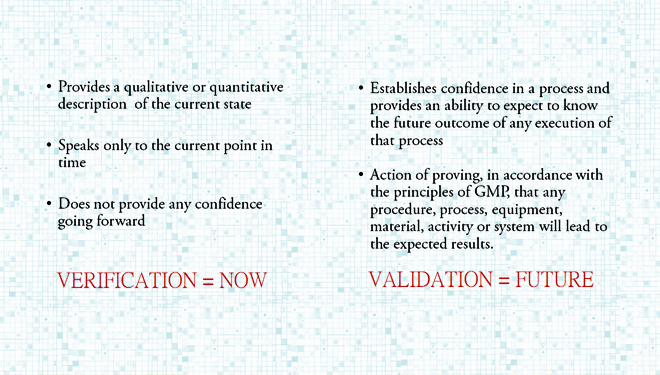
Design and Validation – Drawing the Fine Line
Recently, several questions were submitted by readers, seeking clarity on the fine line between the stages of design and validation. While preparing individual answers to each question, we noted that dealing with them as a group presented an opportunity to address fundamental concepts comprehensively. This month’s blog is dedicated to highlighting the very real differences, […]
Cost Cutting and Quality
The last several years have been turbulent for the US pharmaceutical market. Unfavorable economic conditions have hit all sectors of the global economy. Historically, the pharmaceutical industry has been relatively immune to the downswings of the global economic cycle because illness, which provides the opportunities for these pharmaceutical companies to create and produce drugs, never […]
The Biosimilar Guidance Documents; Industry Responds
Regulatory Summary In an effort to incentivize the market place to introduce competitively priced biologics, the Biologics Price Competition and Innovation Act, passed by the Senate in 2009, was signed into Federal Law as part of the Patient Protection and Affordable Health Care Act by President Obama in 2010. Among other components the BPCI Act: […]
Quality Control: Overhead or Profit Center?
I have recently joined Coda’s team of Quality System bloggers. My professional background is rooted in the development and management of Quality Control Laboratories. The experience working to create, export, streamline, maintain, improve, and manage QC labs has not only been enjoyable, but has also given rise to many valuable insights and lessons. With that […]
Risk Management: A Primer for Lean Quality Assurance
In this blog we will examine what is perhaps the most useful tool in the belt of today’s quality professionals: risk management. Critical Terminology Prior to discussing the topic at hand, I like to provide context for the terminology integral to the concept. The first step on the road to effective system development is alignment […]
Supplier Quality and the Total Cost of Poor Quality; Understand the Relationship and Avoid the Shortcuts!
During my two decade career, including a decade of consulting, I have integrated with a multitude of organizations, a wide variety of cultures, and a broad spectrum of projects, each with unique objectives, team members, and needs. This diversity of experience has been its own reward and continues to incentivize me to forego the obvious […]
The Industrial Revolution: Redux
Market reports indicate that drug and device manufacturers have been adapting traditional models, trending toward heavier usage of contracted suppliers of critical functions such as manufacturing (CMOs) and research (CROs). Trends suggest that this will continue over the next decade. A review of figures for the last decade reveal that outsourced manufacturing by pharma and […]
Understanding, Measuring, and Controlling the total Cost of Quality; The Holy Grail of The Modern Quality System
As a follow up to our Lean article, we decided to continue the discussion of concepts, principles, and tools that aim to reduce operating costs while promoting and improving consumer-centric quality. Among this family of principles are those referred to as quality costing analysis models, or “Cost of Quality” (CoQ) models. Industry has been attempting […]
Is Lean Still a Good Idea?
Recently, a colleague who is a college instructor noted that one of his students asked, “Is Lean still a good idea?” This prompted the teacher to solicit opinions from those of us that toil each day in the world of manufacturing. I typed a short reply. As I was typing, I realized that while the […]
The One Voice Mission: A Quality Opus
I have worked within and around the life sciences community of Quality professionals for more years than I am ready to admit in a public post. Early on in my career, I listened avidly to every experienced person that I worked with, eager to take in everything anyone had to teach me. I was the […]
Corrective and Preventative Action Programs, Beyond Deviation Management
Too often Corrective and Preventative Action (CAPA) programs are considered solely with regard to Deviation Management programs. Modern Quality Systems, including CAPA programs, have been a focus of international harmonization efforts and the FDA’s 21st Century Initiative Program. Both initiatives seek to align the industry, and promote aggressive, proactive programs that drive toward continual process […]

 NAVIGATION
NAVIGATION